PPI Overview
 Proton pump inhibitor drugs (PPIs) effectively halt gastric acid production in the stomach. For this reason, doctors primarily recommend them to treat heartburn or gastroesophageal reflux disease (GERD) symptoms. They also treat conditions associated with acid over-production, such as ulcers, Zollinger-Ellison Syndrome, and erosive esophagitis. Most patients can generally tolerate short-term PPI therapy courses lasting 4-12 weeks. However, many people now take various PPI drugs for occasional acid reflux or heartburn. This over-use led to a rise in serious side effects, including some life-threatening complications like stomach cancer. Learn more about these potential dangers in our PPI overview below.
Proton pump inhibitor drugs (PPIs) effectively halt gastric acid production in the stomach. For this reason, doctors primarily recommend them to treat heartburn or gastroesophageal reflux disease (GERD) symptoms. They also treat conditions associated with acid over-production, such as ulcers, Zollinger-Ellison Syndrome, and erosive esophagitis. Most patients can generally tolerate short-term PPI therapy courses lasting 4-12 weeks. However, many people now take various PPI drugs for occasional acid reflux or heartburn. This over-use led to a rise in serious side effects, including some life-threatening complications like stomach cancer. Learn more about these potential dangers in our PPI overview below.
PPI Overview: Nexium vs. Prilosec
Two of the most popular (and profitable) PPI medications include Prilosec® (omeprazole) and Nexium® (esomeprazole). AstraZeneca originally manufactured, marketed and patented both drugs. Prilosec came first, winning the Food and Drug Administration’s approval in 1989. Just before Prilosec’s patent expired in 2001, AstraZeneca began aggressively marketing its newest PPI, Nexium. Advertising stated Nexium was superior for treating acid reflux symptoms, dubbing it “the little purple pill.” As widespread PPI use increased, patients using these powerful antacids for more than a year reported severe side effects. Since many people reported debilitating health conditions after prolonged PPI use, advocacy groups petitioned the FDA to add a “black box warning” to drugs like Nexium and Prilosec in 2011. This way, they reasoned, the public would be better informed about any PPI medication’s potential risks.
PPI Overview: Adverse Reactions Linked to Long-term Use
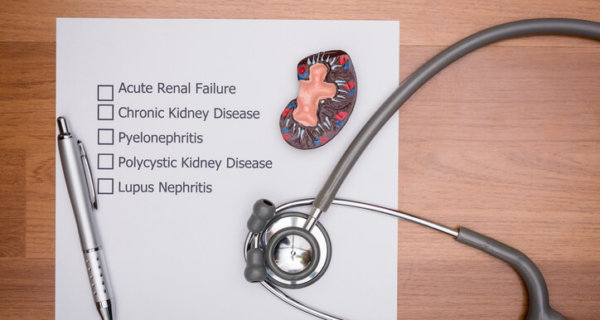
In 2007, researchers published the first medical study showing serious side effects from long-term PPI use. During autopsy, doctors found PPI patients had an increased rate of acute interstitial nephritis (AIN) at death. In 2010, the FDA released a safety communication due to studies showing higher risks of hip, wrist and spine fractures among high-dose users as well as patients taking PPIs for more than two years. The FDA released additional warnings in 2011 and 2012 linking prolonged Nexium and Prilosec use to low magnesium levels as well as increased Clostridium Difficile-Associated Disease (CDAD). In 2014, the FDA updated all standard packaging and labels for Prilosec (omeprazole) and Nexium (esomeprazole). This was done to disclose the risk of serious bacterial infection and C. Diff-related diarrhea, vitamin B12 deficiency, magnesium depletion, kidney inflammation and acute kidney injury.
PPI Overview: Research On Cardiovascular and Kidney Damage Risks
Yet another 2014 study found among 133 patients with biopsy-proven AIN, 70% were drug-induced cases — with omeprazole being most prevalent (12%). Researchers at Stanford University published findings in April 2016 showing older PPI patients had increased heart attack risk. Then a January 2016 study revealed higher risk of chronic kidney disease (CKD) in patients taking PPIs twice daily. A few months later, an American Society of Nephrology study revealed long-term PPI use may increase kidney failure and chronic kidney disease risks. In early 2016, German researchers found a 44% increased dementia risk in elderly patients.
PPI Overview: Lawsuits Filed Against AstraZeneca
Pharmaceutical company AstraZeneca (maker of Prilosec/omeprazole and Nexium/esomeprazole) settled one class-action lawsuit for $20 million in 2013. Then, the company settled a U.S. Justice Department lawsuit in 2015 for $7.9 million. Yet another 2013 lawsuit accused the drug maker of engaging in deceptive marketing practices. Despite medical studies showing no difference in efficacy between PPI formulations, the company heavily promoted Nexium as superior for heartburn relief. Later, a 2015 U.S. Justice Dept. case accused AstraZeneca of participating in a kickback scheme with Medco Heath Solution. Allegations say this was done in order to maintain Nexium’s “sole and exclusive status” within its formularies, violating the False Claims Act. AstraZeneca’s out-of-court settlements in both cases allowed the company to avoid admitting any wrongdoing or going to trial.
PPI Overview: Lawsuits Filed Against Pfizer, Inc.
Pfizer, Inc. (manufacturer, Protonix/lansoprazole) paid even higher out-of-court settlements over the past decade. First, Pfizer settled a claim in 2013 for $55 million over allegations of engaging in off-label marketing for Protonix (pantoprazole). This lawsuit accused Pfizer of marketing Protonix for off-label uses. (Specifically, Protonix was FDA-approved only for treating erosive esophagitis caused by GERD diagnosed by endoscopy). Then, Pfizer settled a second claim in 2016 for $785 million over health care fraud allegations. Lawyers accused the company of charging Medicaid providers a higher price than it offered to private hospitals for purchasing Protonix I.V. and Protonix Oral bundled together at a discounted rate. Moreover, this practice violated the False Claims Act under a “pricing scheme,” according the U.S. Attorney Carmen M. Ortiz.
PPI Overview: Many PPI Drug Injury Cases Are Still Pending
Due to adverse effects from long-term PPI use, many injured patients are suing the drugs’ manufacturers. Claims from consumers and health care providers alike name AstraZeneca, Pfizer, Inc. and other pharmaceutical companies as defendants. Despite the FDA updating various PPI warning labels in mid-2014, many people regularly taking these drugs without a doctor’s supervision may have experienced severe side effects.
Thousands of injured consumers already filed lawsuits seeking justice and compensation for their injuries. Patients managing frequent heartburn symptoms should limit routine PPI medication intake to no more than 4-8 weeks (as recommended by most doctors). Consumers may also choose alternative heartburn remedies, such as H2 blockers, which include fewer serious side effects.
Check your eligibility for compensation.
If you or a loved one suffered from serious complications while taking Nexium, Prilosec or another PPI medication, you may qualify for compensation from the manufacturer. Request your free case evaluation now to see if you may qualify.