Stryker Hip Replacement Settlement & Financial Compensation Information
 Stryker Corporation, which manufactures modular hip replacement systems, faces mounting litigation over defective LFIT CoCr V40™ femoral head implants. Current plaintiffs’ injuries are strikingly similar to those who reached a Stryker hip replacement settlement with the manufacturer back in 2014. No courts have yet awarded a Stryker hip replacement settlement. However, all current hip replacement injury claims involve interchangeable components using the same proprietary titanium alloy. For this reason, previous settlements may prove influential in how Stryker chooses to handle pending product liability claims.
Stryker Corporation, which manufactures modular hip replacement systems, faces mounting litigation over defective LFIT CoCr V40™ femoral head implants. Current plaintiffs’ injuries are strikingly similar to those who reached a Stryker hip replacement settlement with the manufacturer back in 2014. No courts have yet awarded a Stryker hip replacement settlement. However, all current hip replacement injury claims involve interchangeable components using the same proprietary titanium alloy. For this reason, previous settlements may prove influential in how Stryker chooses to handle pending product liability claims.
Stryker Hip Replacement Settlement History
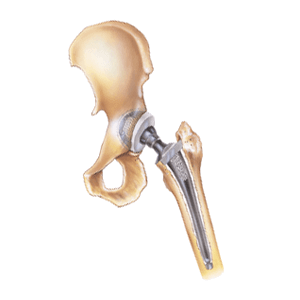
In many traditional metal-on-metal hip replacements, fretting and corrosion eventually results in taper lock failure. Spontaneous dislocation and disassociation of a failed hip implant can be extremely painful and requires immediate revision surgery. In addition, metal blood poisoning (also called metallosis) can produce tumors, bone damage and soft tissue necrosis. Patients with Rejuvenate and ABG II modular-neck stems reported the same metal-on-metal hip replacement side effects. For this reason, Stryker recalled both modular hip implant components in 2012. Two years later, Stryker agreed to pay 4,000 injured Rejuvenate and ABG II plaintiffs a $1.43 billion settlement. Patients whose Stryker Rejuvenate and ABG II modular-neck stems failed due to metal corrosion won about $300,000 each in damages.
Several Stryker LFIT V40 Lawsuits Are Still Pending
Injured LFIT V40 patients started filing lawsuits in 2014, but no plaintiff has yet won a Stryker hip replacement settlement. At least five New Jersey plaintiffs with Accolade TMZF hip stems and LFIT V40 femoral heads filed lawsuits that May. They allege that Stryker failed to adequately warn consumers about metal toxicity and corrosion risks from their hip implant components. Plaintiffs say Stryker hip implants shed microscopic chromium and cobalt particles within the body, which echoes previous metallosis lawsuit claims.
Just one month before the 2016 LFIT V40 recall, Annah Marie Gidora filed another product liability lawsuit against Stryker. Gidora had total hip replacement surgery in January of 2014, then needed emergency revision surgery less than two years later. Gidora says she was permanently injured when her hip replacement system (including the Stryker LFIT V40 femoral head) catastrophically failed. Gidora’s claim accuses Stryker of:
- Negligence
- Defective device manufacturing and design
- Inadequately warning consumers about potential hip implant risks
- Breach of warranty
- Violating New York’s consumer protection laws
And in November 2016, a Massachusetts man with implant corrosion and metallosis filed yet another lawsuit against Stryker. William D’Orlando’s artificial hip replacement system included the Stryker Accolade TMZF hip stem and LFIT V40 femoral head components. D’Orlando claims Stryker’s V40 head and Accolade hip implant designs are defective, and that all similar components should be recalled. Interestingly, Rejuvenate, ABG II and LFIT V40 hip implant components are all made from the same TMZF titanium alloy.
Current Stryker Hip Replacement Settlement Climate
Despite the similarity in Rejuvenate, ABG II and LFIT V40 lawsuit claims, no Stryker hip replacement settlement has yet been reached. If either you or a loved one received a Stryker LFIT V40 femoral head and needed revision surgery a few years after implantation, you may have a case. To see if you may qualify for financial compensation, click the button below now to start your free claim evaluation. In less than two minutes, we check your eligibility for a cash settlement from the device’s manufacturer. Once you’ve completed your case review, a qualified attorney will call to discuss how to get the compensation and justice you deserve.
Check your eligibility for compensation.
If you or a loved one experienced life-threatening complications after Stryker hip replacement surgery, you may qualify for compensation from the manufacturer. Request your free case evaluation now to see if you may qualify.