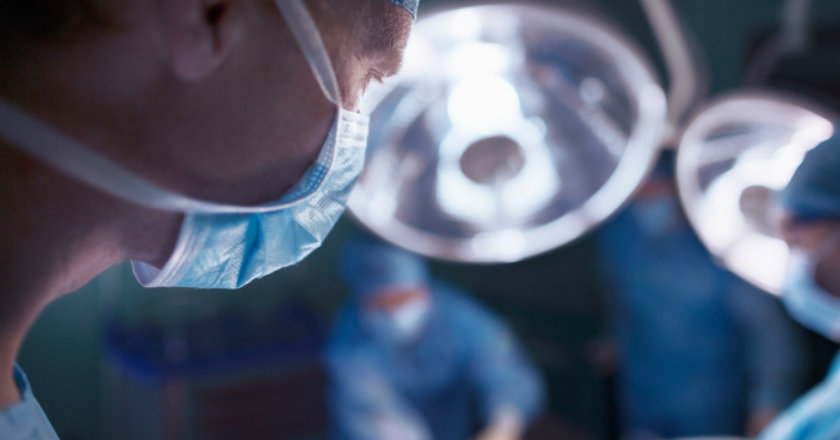
Countless women have needed travsvaginal mesh (TVM) removal surgery following serious and life-threatening complications after having this device implanted to repair pelvic organ prolapse (POP) or stress urinary incontinence.


Countless women have needed travsvaginal mesh (TVM) removal surgery following serious and life-threatening complications after having this device implanted to repair pelvic organ prolapse (POP) or stress urinary incontinence.
Numerous transvaginal mesh (TVM) lawsuits have been filed against the manufacturer Ethicon, Inc., and the federal litigation established in the U.S. District Court, Southern District of West Virginia continues to proceed.
Another transvaginal mesh lawsuit has been filed against the manufacturer American Medical Systems Inc., in the Southern District of West Virginia.
The lawsuit settlement funding company Legal-Bay LLC announced on June 4, 2014, a new lawsuit funding programs for women with proposed settlement cases against American Systems transvaginal mesh lawsuits.
Lawsuits against manufacturers of transvaginal mesh devices such as Johnson & Johnson and Endo International have been making headlines lately for the ongoing cases and settlements with thousands of women who filed suits after suffering serious and often life-threatening complications due to transvaginal mesh.
Transvaginal mesh has been used in countless surgeries to repair pelvic organ prolapse (POP), but in many cases it has led to further damage, serious complications and the need for even more surgeries.

The U.S. Food and Drug Administration released a statement announcing two proposals addressing the serious health risks linked to surgical mesh used for transvaginal repair following a pelvic organ prolapse (POP).
Endo Health Solutions, which bought American Medical Systems (AMS) Inc., in 2011, reached a settlement this week in cases of women suing over implanted transvaginal mesh, according to the Charleston Gazette. About 6,000 women will be affected by this settlement, which came to about $830 million.
Endo International PLC., the parent company of American Medical Systems (AMS), was issued a letter of warning from the U.S. Food and Drug Administration (FDA) following an inspection of the facility where it found three federal regulation violations against how some urological devices and transvaginal mesh are manufactured.

A pelvic organ prolapse, or POP, occurs when pelvic organs droop or descend.