Xarelto Lawsuit Information for Blood Thinner Patients
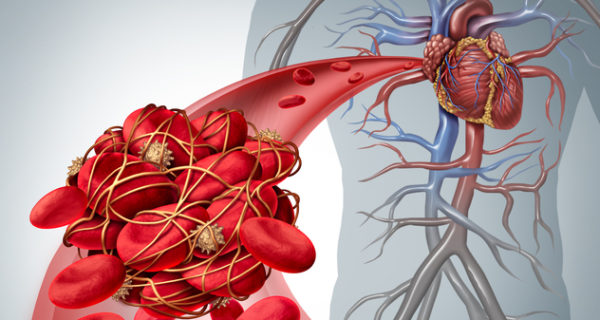
 Popular prescription anticoagulant Xarelto® belongs to a newer drug class called Factor Xa inhibitors. These drugs don’t require monthly blood monitoring, unlike warfarin. The U.S. Food and Drug Administration initially approved Xarelto (rivaroxaban) in July 2011. Within three years, injured patients sued the drug’s makers for failing to warn them about potentially life-threatening side effects. If you developed blood clots or uncontrolled internal bleeding while taking Xarelto, you may qualify for financial compensation. Families may also file a wrongful death lawsuit on any deceased Xarelto victim’s behalf. Review more Xarelto lawsuit information below to see if you may have an eligible claim.
Popular prescription anticoagulant Xarelto® belongs to a newer drug class called Factor Xa inhibitors. These drugs don’t require monthly blood monitoring, unlike warfarin. The U.S. Food and Drug Administration initially approved Xarelto (rivaroxaban) in July 2011. Within three years, injured patients sued the drug’s makers for failing to warn them about potentially life-threatening side effects. If you developed blood clots or uncontrolled internal bleeding while taking Xarelto, you may qualify for financial compensation. Families may also file a wrongful death lawsuit on any deceased Xarelto victim’s behalf. Review more Xarelto lawsuit information below to see if you may have an eligible claim.
Xarelto Lawsuit Information: What Is Xarelto?
Bayer and Janssen Pharmaceuticals, a Johnson & Johnson subsidiary company, both manufacture rivaroxaban (Xarelto). Xarelto is extremely popular with U.S. consumers due to its ability to treat a variety of medical issues, including:
- reducing risk of blood clots as well as strokes in people with atrial fibrillation
- treating deep vein thrombosis (DVT) as well as pulmonary embolism (PE)
- lowering blood clot risks for hip or knee replacement surgery patients
Xarelto suffered some June 2013 backlash from the FDA due to “misleading claims” about the drug’s efficacy and associated risks. In fact, the FDA’s letter warns J&J about minimizing Xarelto’s risks in a 2013 ad found in WebMD magazine. One year later, Ruth E. McGowan filed the first wrongful death Xarelto lawsuit on behalf of her father, Thomas C. Dunkley. In 2014, Louisiana Judge Eldon E. Fallon approved a class-action filing to consolidate several thousand federal Xarelto claims. Serious Xarelto side effects include uncontrolled internal bleeding, pulmonary embolism, deep vein thrombosis — and in some cases, even death. At 17.5% of the U.S. prescription blood thinner market, Xarelto remains popular with doctors and patients alike. And that’s despite no reversal agent to save patients’ lives from uncontrolled bleeding episodes. Then in May 2018, the FDA finally approved a Xarelto antidote called AndexXa that also works equally well on Eliquis patients.
Xarelto Lawsuit Information: Allegations Similar to Eliquis, Pradaxa Claims
Janssen now faces mounting negligence accusations from Xarelto patients across the U.S. And that’s in addition to wrongful death claims from victims’ surviving family members. The manufacturer allegedly failed to warn consumers about all potential side effect risks, echoing the FDA’s previous concerns. Reviewing Xarelto lawsuit information shows many plaintiffs filing failure to warn claims against the drug’s makers. Allegations, plaintiffs’ names as well as other relevant Xarelto lawsuit information, is available at the multi-district litigation’s website.
Notably, this isn’t the first time a new wave blood thinner medication drew public scrutiny. Another anticoagulant called Pradaxa (dabigatran) reached a $650 million settlement over similar claims in 2014. Both Xarelto and another blood thinner medication, Eliquis, face near-identical product liability lawsuits. Plaintiffs researching Eliquis, Pradaxa or Xarelto lawsuit information may see similar allegations in all three drug injury cases, since they’re all newer anticoagulants.
Xarelto Lawsuit Information: Bayer, J&J Agree to Settle 25,000 Pending Claims for $775 Million
In March 2019, both companies finally agreed to settle all 25,000 Xarelto lawsuits still pending in federal and state courts. While Bayer as well as J&J didn’t admit any liability, they chose to split the settlement payments down the middle. Both companies issued a statement saying they agreed to settle in order to avoid costly ongoing Xarelto litigation. Injured plaintiffs have a limited time window to file their claim to qualify for a significant cash settlement. To see if you may qualify for financial compensation, click the button below and complete your online claim evaluation in less than two minutes!
Check eligibility for compensation.
If you or a loved one experienced blood clots, uncontrolled bleeding or death while taking Xarelto, you may qualify for financial compensation from the manufacturer. Request your free case evaluation now to see if you may qualify.
Lori Polemenakos is Director of Consumer Content and SEO strategist for LeadingResponse, a legal marketing company. An award-winning journalist, writer and editor based in Dallas, Texas, she's produced articles for major brands such as Match.com, Yahoo!, MSN, AOL, Xfinity, Mail.com, and edited several published books. Since 2016, she's published hundreds of articles about Social Security disability, workers' compensation, veterans' benefits, personal injury, mass tort, auto accident claims, bankruptcy, employment law and other related legal issues.